Flame Photometry and its applications
Flame photometry is a process wherein the emission of radiation by neutral atoms is measured.
The neutral atoms are obtained by the introduction of the sample into the flame. Hence the name flame photometry.
Since radiation is emitted, it is also called flame emission spectroscopy.
Currently, it has become a necessary tool in the field of analytical chemistry. Flame photometer can be used to determine the concentration of certain metal ions like sodium, potassium, lithium, calcium and cesium etc. In flame photometer spectra the metal ions are used in the form of atoms.
1. Flame photometer working principle
When a solution of metallic salt is sprayed as fine droplets into a flame. Due to the heat of the flame, the droplets dry leaving a fine residue of salt. This fine residue converts into neutral atoms.
Due to the thermal energy of the flame, the atoms get excited and after that return to the ground state. In this process of return to the ground state, excited atoms emit radiation of a specific wavelength. This wavelength of radiation emitted is specific for every element.
This specificity of the wavelength of light emitted makes it a qualitative aspect. While the intensity of radiation depends on the concentration of the element. This makes it a quantitative aspect.
The process seems to be simple and applicable to all elements. But in practice, only a few elements of Group IA and Group IIA (like Li, Na, k & Ca, Mg) are only analyzed.
The radiation emitted in the process is of a specific wavelength. Like for Sodium (Na) 589nm yellow radiation, Potassium 767nm range radiation.
2. Parts of flame photometer
A simple flame photometer consists of the following basic components:
Source of flame: A Burner in the flame photometer is the source of flame. It can be maintained in at a constant temperature. The temperature of the flame is one of the critical factors in flame photometry.
Nebulizer: Nebulizer is used to send homogeneous solution into the flame at a balanced rate.
Optical system: The optical system consists of convex mirror and convex lens. The convex mirror transmits the light emitted from the atoms. Convex mirror also helps to focus the emissions to the lens. The lens helps to focus the light on a point or slit.
Simple color filters: The reflections from the mirror pass through the slit and reach the filters. Filters will isolate the wavelength to be measured from that of irrelevant emissions.
Photo-detector: The intensity of radiation emitted by the flame is measured by photo detector. Here the emitted radiation is converted to an electrical signal with the help of photo detector. These electrical signals are directly proportional to the intensity of light.
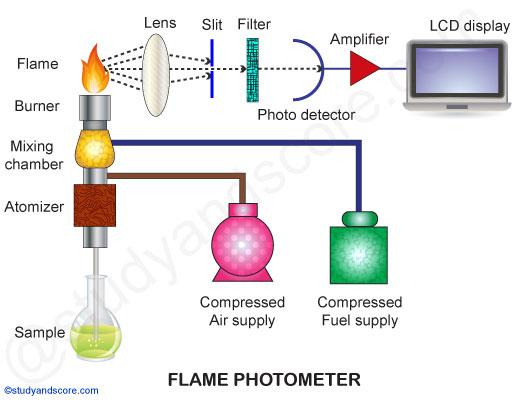
Fig 1.2
3. Flame photometer uses/ Applications
1. For qualitative analysis of samples by comparison of spectral emission wavelengths with that of standards.2. For quantitative analysis to determine the concentration of group IA and IIA elements. For example,
a) Concentration of calcium in hard water.
b) Concentration of Sodium, potassium in Urine
c) Concentration of calcium and other elements in bio-glass and ceramic materials.
4. Advantages of flame photometer
- The method of analysis is very simple and economical.
- It is quick, convenient, selective and sensitive analysis.
- It is both and qualitative and quantitative in nature.
- Even very low concentrations (parts per million/ppm to parts per billion/ppb range) of metals in the sample can be determined.
- This method compensates for any unexpected interfering material present in the sample solution.
- This method can be used to estimate elements which are rarely analyzed.
5. Disadvantages of flame photometer
- In spite of many advantages, this analysis technique has quite a few disadvantages:
- The accurate concentration of the metal ion in the solution cannot be measured.
- It cannot directly detect and determine the presence of inert gases.
- Though this technique measures the total metal content present in the sample, it does not provide the information about the molecular structure of the metal present in the sample.
- Only liquid samples may be used. Also sample preparation becomes lengthy in some cases.
- Flame photometry cannot be used for the direct determination of each and every metal atom. A number of metal atoms cannot be analyzed by this method. The elements such as carbon, hydrogen and halides cannot be detected due to their non-radiating nature.
Very informative and nicely put together.
ReplyDelete